We undertake projects in areas where we have tremendous experience

Partnerships
Amay has clients & partners across globe in the Bio-Pharma industry. We help our clients increase sales and establish partnerships.
Industries
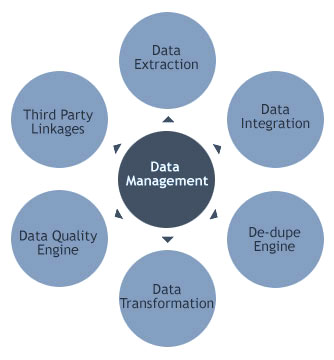
Extensive experience in clinical trial data management and regulatory submissions around the globe

Increased sales
We help our clients with research, relationship building, introduction to new business opportunities & follow-up.

Efficiency
We built Innovative tools to improve the efficiency of clinical trial conduct saving time and money to sponsors.
Welcome to - is improving efficiency of clinical trial management - saving time and money for our clients !!
Contact Now Contact NowAbout Amay Inc
We help our clients in the Bio-Pharmaceutical industry with Innovative products for efficiency improvement of clinical trial management.
Our tools focus on PV Intake & reporting, Statistical programming, Regulatory Agency (FDA, PMDA, EMA) submission deliverables like SDTM, ADAM, aCRF, eCRF, define.xml, SDRG, CSR, TLF with quality while keeping cost down with innovation.
Amay is helping clients run more efficient with process improvement. We do this by providing organizations the insights and capabilities they need to balance the introduction of new, innovative solutions while continuing to maintain ongoing operations in line with cost and quality expectations.
Amay Inc., supports clients globally from offices in USA and India. We have satisfied clients with our work ethics and ability to achieve results. Some of our clients have global footprint.

Amay provided services:
- Training & Support Amay’s Tools for Pharmacovigilance and statistical programming
- Clinical study data management programming deliverable generation for ongoing studies
- Study closeout activities
- Regulatory Submission deliverable generation (FDA, PMDA, EMA)
- Staff augmentation for Clinical statistical programming activities
- Pharmacovigilance Intake and reporting services

